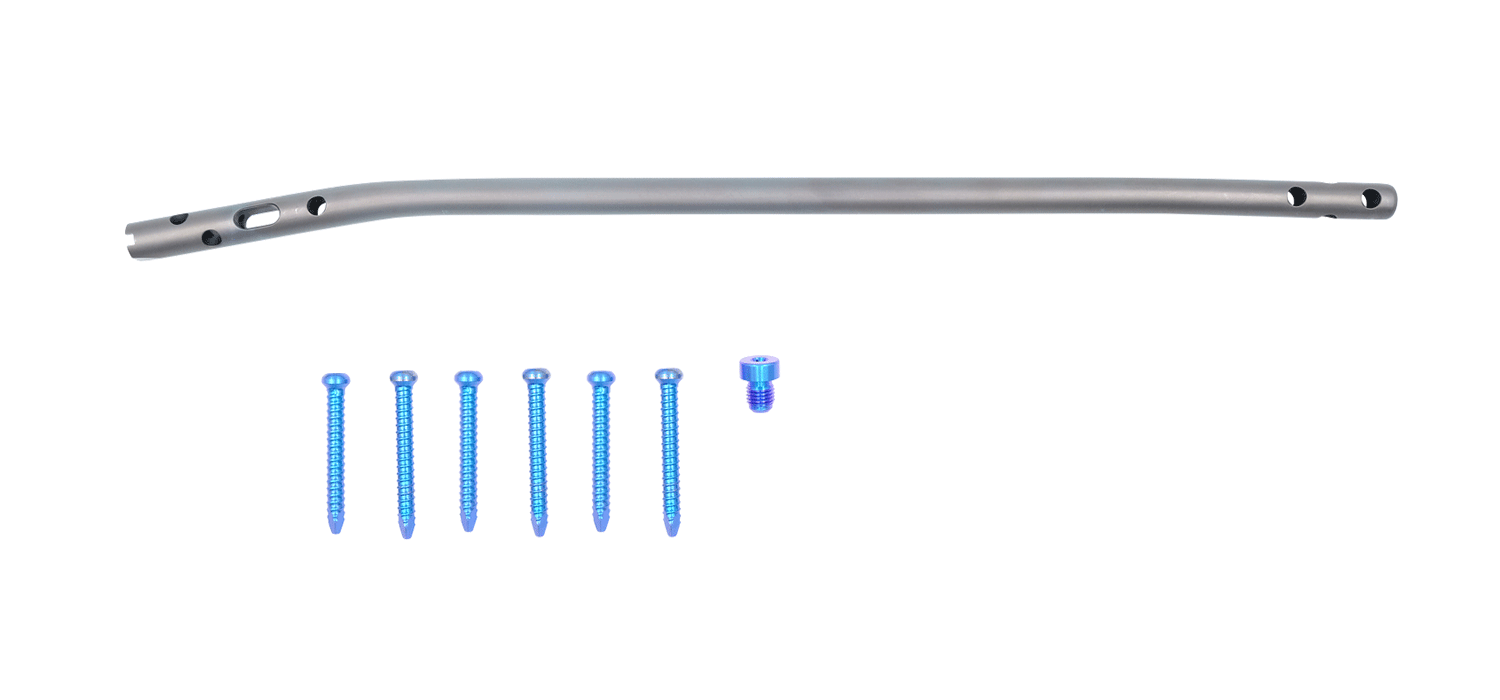
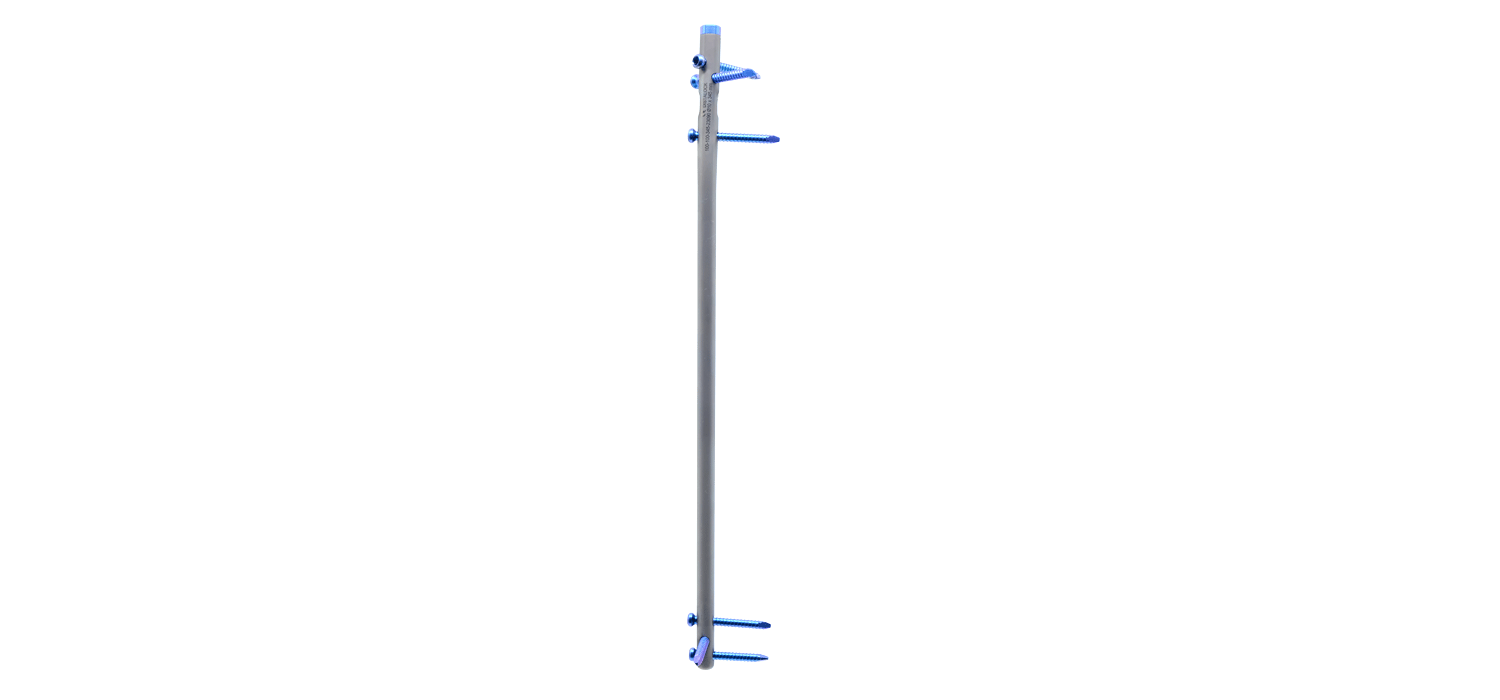
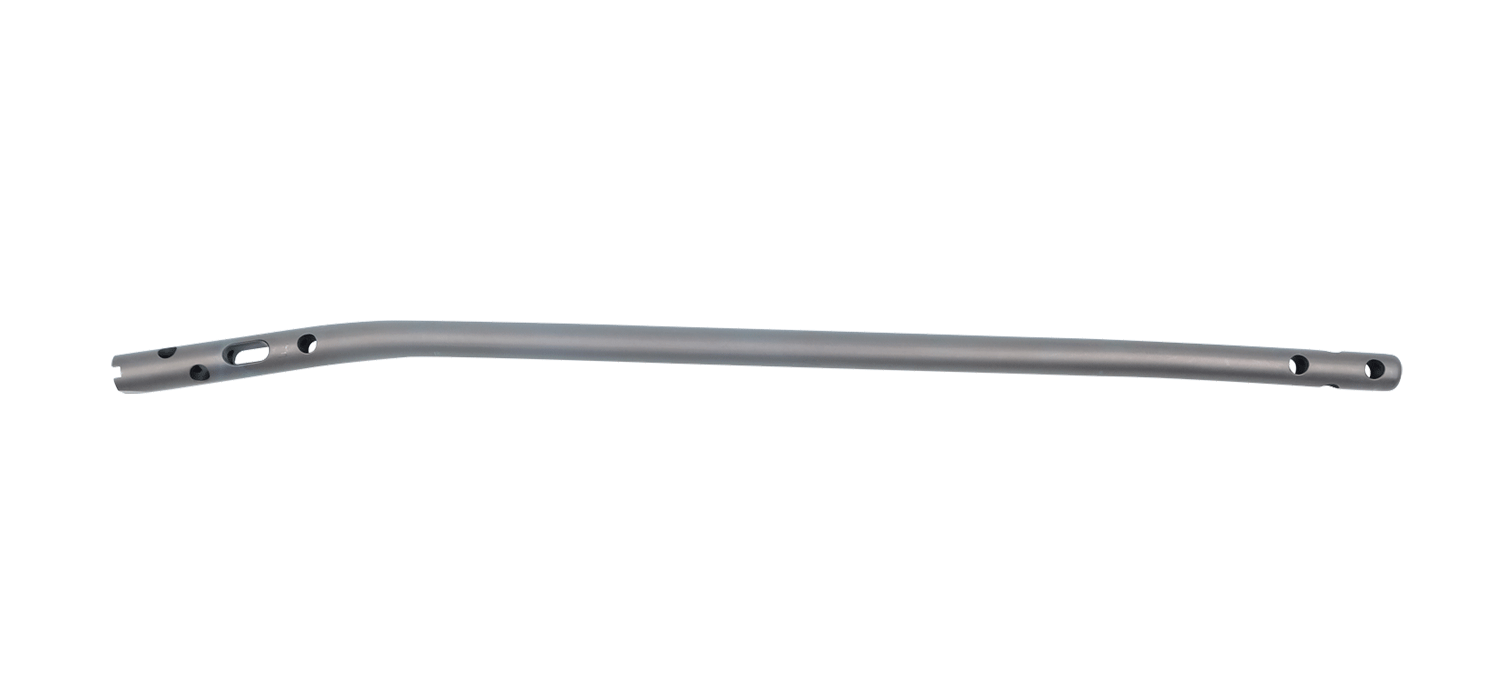
Tibia Intramedullary Nail System
Tibial Intramedullary Nails: They are used in proximal, distal, and shaft fractures of
the tibia bone. It has a cannulated and circular cross-section with curves, featuring 3
distal and 4 proximal holes through which the locking screws pass. The Tibia
Intramedullary Nail has a 10° proximal offset angle and a 3° distal offset angle. The 3
distal holes are for static locking only. One of the 4 proximal holes is a slot designed
for either dynamic or static locking.
| Ürün Adı | Tibia Intramedullary Nails; |
| Ürün Tanımı | It is a structure with bends, featuring a cannulated, circular cross-section and three distal holes for locking screws and four proximal holes. |
| Ürün Kullanım Amacı | The Tibia Intramedullary System has a proximal bend of 10° and a distal bend of 3°. |
| Endikasyonlar | Tibia intramedullary nails come in various lengths. |
| Kontraendikasyonlar | They come in lengths ranging from 255mm to 420mm, increasing in 15mm increments. |
| Hasta Popülasyonları | These lengths are available in each diameter of 9mm, 10mm, and 11mm |
| Ürün Komplikasyonları | The 3 distal holes are for static locking only. |
| Temas Edebileceği Organlar | One of the proximal holes is rectangular in shape, which is intended for dynamic locking. |
| Vücutla Temas Süresi | There is a female thread at the proximal end of the nail. This is provided for the implantation and removal of the nail. |
| Sterilite | Additionally, this female thread is for the peak screw socket. |